Introducing the Boson Antigen Covid Test: Rapid Covid Antigen Test Card
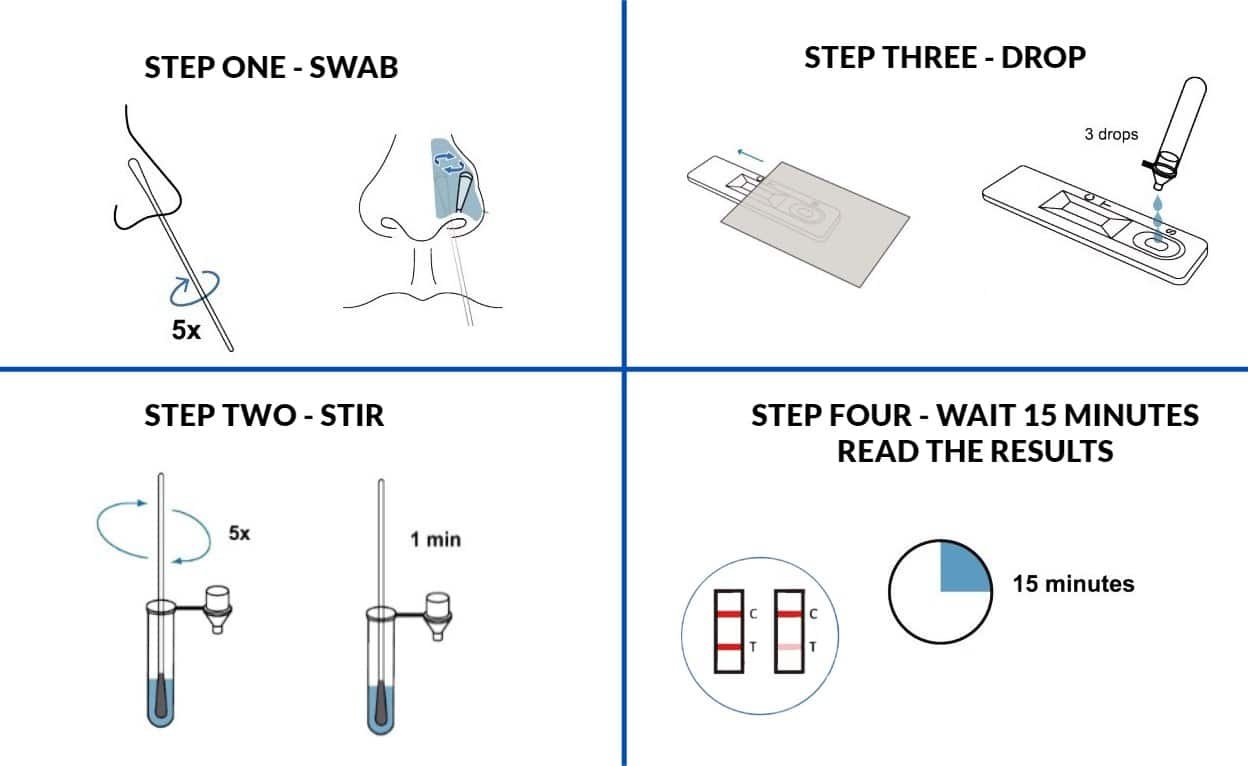
Welcome to the forefront of rapid and reliable COVID-19 detection with the Boson Rapid Home Antigen Covid Test Card. This user-friendly kit is designed for the swift identification of SARS-CoV-2 antigens in anterior nasal specimens, providing a valuable tool for emergency use authorization (EUA) purposes.
Key Features:
- Rapid Results: Experience quick and efficient results for on-the-spot insights.
- In vitro Diagnostic Use: Ensuring accuracy in diagnostic applications.
Important Information:
- Emergency Use Authorization (EUA) Only: Specifically authorized for emergency use to address critical situations.
- For More Information on EUAs: Visit the FDA’s Emergency Use Authorization information page here.
- COVID-19 Updates: Stay informed with the latest information on COVID-19 by visiting CDC`s Covid information.
Harness the power of the Rapid Covid Antigen Test Card from Boson for accurate and timely results in the detection of SARS-CoV-2 antigens. For emergency use authorization purposes and in vitro diagnostic use only.











Reviews
There are no reviews yet.